Condensed Electron Configuration For Kr
First: Decide the number of electrons. Krypton has a total of 36 electrons
Second: KNOW YOUR ORBITALS
Know how many electrons each orbital tin concur and their guild.
(Refer to the following pictures as notes)
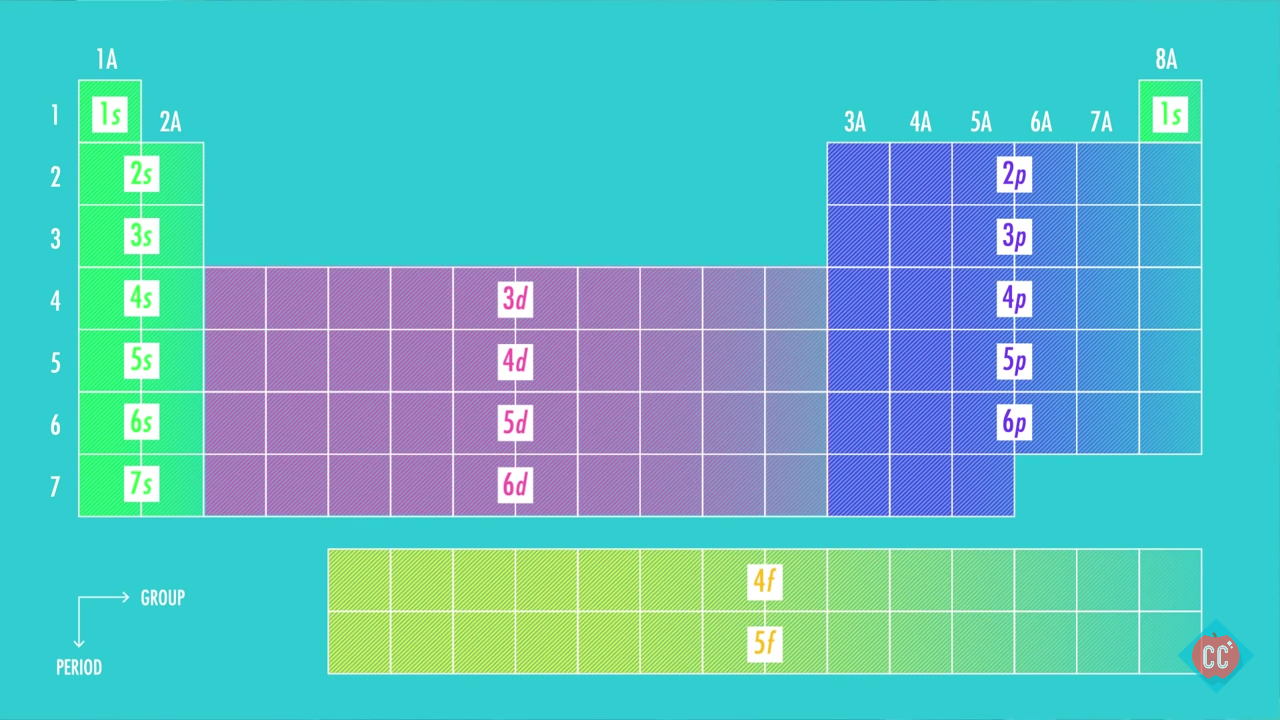
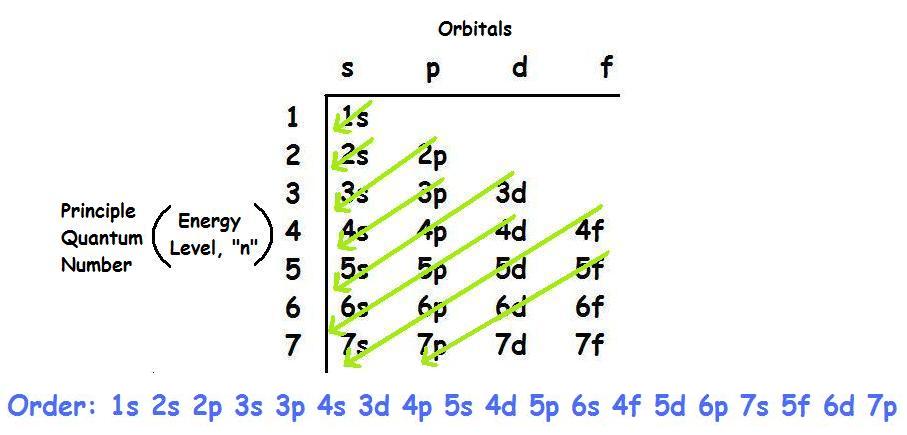
3rd: Write out the electron configuration
For Krypton and about of the elements there are more the just 1 fashion (usually 2) to write the electron configuration. One manner is to write out the unabridged electron configuration past going through each orbital or we tin use a shorthand note using the noble gases as a starting bespeak. I volition get through both methods:
First Method: (Long way)
We know that Krypton has
Nosotros know that the
2d Method (Shorthand)
The key to using this method is to place the element of group 0 closest to the desired element that is at a lower energy (Has a lower atomic number if I'm loosely speaking). In essence, the shorthand note tells us the configuration by using a noble gas element equally our starting point instead of starting all the way at the
Coincidently, Krypton itself is a noble gas so we could write the electron configuration as
*Notice how the configuration
All in all, the three given answers are right means of figuring out the footing-state electron configuration of Krypton .
Condensed Electron Configuration For Kr,
Source: https://socratic.org/questions/what-is-the-ground-state-electron-configuration-of-the-element-kr
Posted by: leewhapin.blogspot.com


0 Response to "Condensed Electron Configuration For Kr"
Post a Comment